Abstract

INTRODUCTION: Patients receiving autologous chimeric antigen receptor T-cell (CAR-T) therapies targeting B-cell maturation antigen (BCMA) for multiple myeloma (MM) may require bridging therapy before CAR-T infusion to maintain some level of disease control. Many bridging regimens used in MM include alkylating agents such as cyclophosphamide (Cy), for example V(T)D-PACE, DCEP, or modified hyperCVAD. However, there is no consensus around the optimal choice or chemotherapy dose intensity of such bridging regimens.
METHODS: We performed a single-center analysis of all instances of bridging therapy before planned autologous BCMA CAR-T during a 5-year period ending March 2022. We classified bridging regimens into three cohorts: (1) IV dose-dense Cy (IV_ddCy) with inpatient IV Cy every 12-24 hours and mesna support per modified hyperCVAD protocol (Narayan 2020); (2) Weekly_Cy, with weekly oral/IV Cy; and (3) No_Cy, where Cy was not used in bridging. Demographic and disease-related characteristics at time of T-cell collection, as well as post-CAR-T outcomes for patients who received CAR-T, were compared using Fisher's and Kruskal-Wallis testing. Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan-Meier method.
RESULTS: There were 69 discrete instances of bridging therapy among 63 unique patients, 89% of whom were previously Cy-exposed. Of these 69 instances, 38% involved IV_ddCy (n=26), 33% involved Weekly_Cy (n=23), and 29% involved No_Cy (n=20). Dexamethasone was used in most instances (88%, n=61) while 12% of instances incorporated radiation (n=8). Age, number of prior lines, triple-class refractory status, presence of high-risk cytogenetics, serum levels of paraprotein or involved light chains, bone marrow plasma cell burden, presence of renal dysfunction, rates of ≥PR to bridging, and rates of PD to bridging were similar between cohorts. However, extramedullary disease (EMD) was borderline more common with IV_ddCy (26%, n=6) than with Weekly_Cy (5%, n=1) (p=0.06). IV_ddCy bridging was associated with a higher total Cy exposure than Weekly_Cy: median 2100 vs 615 mg/m2 (p<0.01).
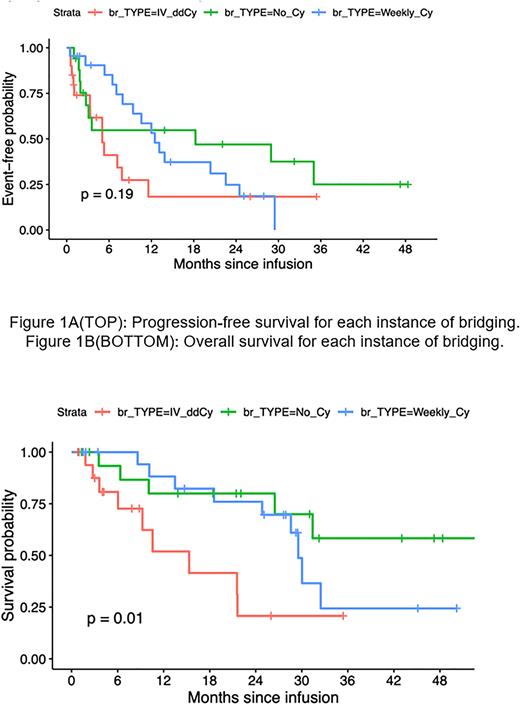
All 10 instances of bridging without subsequent CAR-T were due to manufacturing failures. Among 59 instances of bridging followed by CAR-T, there were no significant differences in vein-to-vein intervals: 45 days with IV_ddCy, 39 days with Weekly_Cy, and 46 days with No_Cy. There were no differences in rates of CRS or ICANS. However, platelet recovery took longer with IV_ddCy than Weekly_Cy (median 64.0 vs 40 days, p=0.02). Median progression-free survival (PFS) was 5.0 months (95% confidence interval [CI] 3.3-not reached [NR]) with IV_ddCy versus 12.5 months (95% CI 9.4-NR) with Weekly_Cy (Figure 1A, p = 0.19). Median overall survival (OS) was 15.3 months (95% CI 9.2-NR) with IV_ddCy versus 29.5 months (95% CI 28.6-NR) with Weekly_Cy (Figure 1B, p = 0.01).
DISCUSSION: In our retrospective analysis, patients bridged with IV_ddCy versus Weekly_Cy had comparable measures of MM burden. IV_ddCy bridging did not significantly prolong vein-to-vein intervals but was associated with delayed platelet recovery, perhaps related to a total median Cy exposure over 3 times that of Weekly_Cy. Importantly, IV_ddCy was associated with worsened OS despite comparable values for most markers of disease aggressiveness (apart from EMD). Study limitations include small sample size as well as confounding from unmeasured markers of MM aggressiveness among patients who were deemed to have required IV_ddCy bridging. However, our analysis suggests that intensive inpatient chemotherapy-based bridging regimens may not offer advantages over weekly Cy-containing regimens for most patients with MM who require pre-CAR-T bridging therapy.
Disclosures
Lo:Oncopeptides: Consultancy; EUSA Pharma: Consultancy. Knoche:Amgen: Honoraria. Chung:Janssen: Research Funding; Cellectis: Research Funding; BMS/Celgene: Research Funding; AbbVie: Research Funding; Merck: Research Funding; Caelum: Research Funding; Sanofi: Honoraria. Wong:Catalent Biologics: Consultancy; Bristol Myers Squibb: Research Funding; Caelum: Research Funding; Genentech: Research Funding; Patient Discovery: Research Funding; Fortis: Research Funding; GSK: Research Funding; Janssen: Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees; Dren Bioscience: Consultancy. Wolf:Adaptive Biotechnologies: Consultancy. Martin:GlaxoSmithKline and Legend Biotech: Consultancy; Amgen, Johnson & Johnson / Janssen, Sanofi, and Seattle Genetics: Research Funding; Legend Biotech: Consultancy. Shah:GSK, Amgen, Indapta Therapeutics, Sanofi, CareDx, Kite, Karyopharm, Oncopeptides,: Consultancy; AstraZeneca: Current Employment, Current equity holder in publicly-traded company; Aztra Zeneca: Current Employment, Other: stock ownership; Celgene/BMS, Janssen, Bluebird Bio, Sutro Biopharma, Teneobio, Poseida, Nektar, Precision Biosciences: Research Funding. Banerjee:Pack Health: Research Funding; SparkCures: Consultancy; Sanofi: Consultancy; Guidepoint Global: Consultancy; Eradigm Consulting: Consultancy; i3 Health: Consultancy; Clinical Care Options: Honoraria; Curio Science: Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal